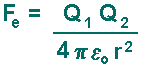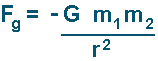Electric Force Coulomb's law, developed in the 1780s by French physicist Charles Augustin de Coulomb, may be stated as follows:
The definition of the Coulomb Force is represented by this equation: The electric force Fe (that acts on charges):
A positive result indicates a repulsive force (two negative charges or two positives will result in that). A negative result indicates an attractive force (one charge of each type will result in that). Comparison of Electic Force to Gravitational ForceThe gravitational force (that acts on masses) is given my the following equation:
(the negative sign shows that the force is always attractive)
An example - comparing the forcesConsider two electrons one metre apart:
The gravitational force acting on the two electrons is 8.3 X 10 -61 N The coulomb force acting on the two electrons is 2.3 x 10-28 N Therefore the coulomb force is 1032 times bigger than the gravitational force 100,000,000,000,000,000,000,000,000,000,000 times bigger .... that's why we have to think of charged particles as being in a 'different dimension' to us.... they experience the 'hills and dales of the electric field' much more strongly than the 'ups and downs' of the gravitational field.... and the effect of gravity on their mass is negligible to the effect of the electric field on their charge. We have much more mass than net charge - so we are often unaware of electric fields - but very aware of gravity!
LOJ MARCH 2002 - revamped 2023 |
Follow me...
|